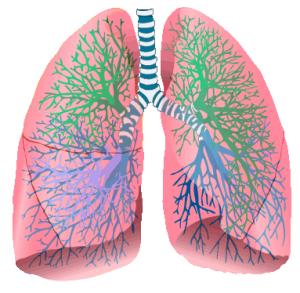
LUNGS “Lungs (animated)” by Mikael Häggström – Image:Respiratory system complete numbered.svg (Public domain licence). Licensed under Public Domain via Wikimedia Commons.
Take a slow, deep breath.
“I am large, I contain multitudes.” ~ Walt Whitman
The physiological mechanisms that make up a single breath could fill an entire book. And yet, for an event that recurs an average of 20,000 times a day, we generally pay very little attention to our breathing (Priban, 1963). In fact, it may be more accurate to say that your breath breathes you, rather than the other way around. We will spend the remainder of this article studying the intricacies of the slow, deep breath that you brought into awareness after reading my first five words.
Before we make our breath conscious, we must begin at the beginning and study how our breath breathes us.
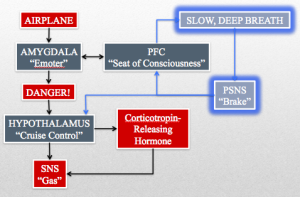
Despite the neuroscientific debate over its evolutionary veracity, Dr. MacLean’s triune brain model will serve as a solid foundation on which to build our understanding of our breath. Dr. Paul MacLean held a dual appointment at Yale Medical School as a professor of psychiatry and physiology before joining the National Institute of Mental Health (NIMH) in 1957. Dr. MacLean first coined the term “limbic system” to describe the set of structures that make up the emotional heart of the brain. MacLean’s limbic system consisted of, among other formations, previously discussed structures such as the amygdala/“Emoter,” the hippocampus/“Memorizer,” the cingulate cortex (the ACC/“Attender” and the PCC/“Emotional Integrator”), the thalamus/“Relay,” and the hypothalamus/“Cruise Control.” (MacLean, 1990)
Following his work with the newly described limbic system, Dr. MacLean went on to outline his evolutionary theory of the human triune brain. The proposed triune brain model consisted of three systems: the reptilian complex (R-complex), the paleomammalian complex, and the neomammalian complex. As is apparent by their names, these systems were thought to represent progressive evolutionary steps up the developmental tree.
Dr. MacLean’s reptilian complex referred to the part of the human brain thought to be a remnant of some of our earliest vertebrate ancestors: the reptiles and birds. The reptilian complex consisted of, among other structures, the basal ganglia/“Pattern Generator” and the brainstem. (MacLean, 1990)
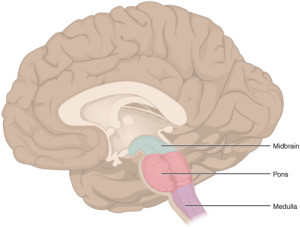
BRAINSTEM “1311 Brain Stem” by OpenStax College – Anatomy & Physiology, Connexions Web site. http://cnx.org/content/col11496/1.6/, Jun 19, 2013.. Licensed under CC BY 3.0 via Wikimedia Commons.
I like to refer to the brainstem as the “central processing unit” (CPU) of the brain because of its similarities with the CPU of a computer. A computer CPU carries out the instructions of a given computer program by performing basic algorithmic operations. So too does the brainstem/“CPU” carry out the instructions of the higher brain structures.
The brainstem/“CPU” provides the basic programming for your heartbeat, breathing pattern, metabolism, sleep-wake cycle, and general alertness. Higher cortical “programs” can augment the output of the brainstem/“CPU” based on environmental need, dialing the aforementioned processes up or down as required. The brainstem/“CPU” also serves as a conduit between the body and the rest of the brain, transmitting vital input and output messages between the two.
The paleomammalian complex represents the second of three steps along the evolutionary path of the triune brain. The paleomammalian complex is synonymous with Dr. MacLean’s limbic system.
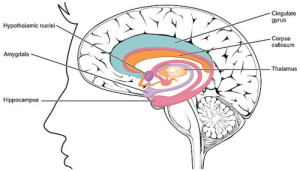
LIMBIC SYSTEM “1511 The Limbic Lobe” by OpenStax College – Anatomy & Physiology, Connexions Web site. http://cnx.org/content/col11496/1.6/, Jun 19, 2013.. Licensed under CC BY 3.0 via Wikimedia Commons.
We highlighted a few of the structures contained with the limbic system in the above paragraphs, but for the purpose of our discussion today we will narrow our focus to the amygdala/“Emoter” and the hypothalamus/“Cruise Control.” These representative structures highlight the function of the limbic system as the source of emotionally motivated behavior. We will revisit these structures in more detail in later paragraphs.
The final evolutionary step along Dr. MacLean’s triune brain paradigm was the neomammalian complex. The neomammalian complex is equivalent to our neocortex. The neocortex is the most evolutionarily recent and complex part of the brain; it gives rise to your conscious experience of the world, your ability to make rational decisions, and, importantly, your capacity to modulate your limbic system and brainstem/“CPU.” The part of the neocortex that is most important for today’s discussion is the prefrontal cortex (PFC). The PFC is located at the most frontal part of the brain, exemplifying the role of the neocortex as the “seat of consciousness”. The PFC is central to decision-making, planning, emotional experience and modulation, and logical thought.
So to review, in order of evolutionary development, Dr. MacLean posited the brainstem/“CPU” (reptilian complex), the limbic system (paleomammalian complex), and the neocortex (neomammalian complex). The stepwise progression of these separate complexes has since been shown to be overly simplistic and relatively evolutionarily inaccurate (Butler & Hodos, 2005). Despite the controversy, the divisions and metaphorical allusions contained within the triune model of the brain can be helpful for the layperson to understand structural and functional characteristics of the brain.
For the purposes of today’s discussion we will focus primarily on the brainstem/“CPU,” the hypothalamus/“Cruise Control” and the amygdala/“Emoter,” and the prefrontal cortex PFC/“Seat of Consciousness”.
After this extended digression, I’d ask that you again pause to take a slow, deep breath. Most of the research papers I will be citing in today’s article come from studies in which “slow” breathing has been operationalized as 6 breaths per minute. Considering that an adult maintains an average respiratory rate of 15 breaths per minute, “slow” breathing requires a 60% reduction of your typical physiology (Priban, 1963). We will come to see that this physiologic gap between typical and “slow” contains the biological roots for a more conscious modulation of your emotional currents.
Why do you breathe?
Very simply, you breathe to absorb oxygen (O2) into your bloodstream and excrete carbon dioxide (CO2). O2 plays a vital role in the production of energy in nearly all living organisms (save for the anaerobes). However, if we ask the same “Why?” question of your neuroanatomy, you will receive a much more complex answer.
![INHALE By LadyofHats (self-made based in my own lungs diagram) [Public domain], via Wikimedia Commons](/wp-content/uploads/2015/02/inhale-300x257.png)
INHALE By LadyofHats (self-made based in my own lungs diagram) [Public domain], via Wikimedia Commons
The diaphragm receives its marching orders from the phrenic nerve. The phrenic nerve receives signals from the cervical spinal cord indicating when to signal the diaphragm to contract and relax. I learned the rhyme “C3, 4, 5, keeps the diaphragm alive” in anatomy class to remind me that the 3rd, 4th, and 5th cervical nerves make up the phrenic nerve.
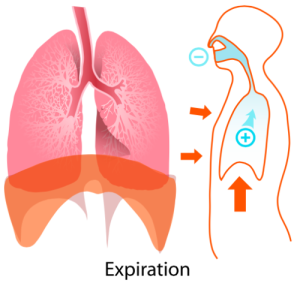
EXHALE “Expiration diagram” by LadyofHats – Own work. Licensed under Public Domain via Wikimedia Commons.
In addition to the diaphragm, muscles of the chest wall, neck, and upper back also participate in inspiration and expiration, but for our purposes we will only examine the diaphragm.
To understand how the phrenic nerve knows when to tell the diaphragm to initiate a breath, we must return to Dr. MacLean’s most primal structure: the brainstem/“CPU.” The brainstem/“CPU” contains groups of neurons (the basic cell of the brain) known as central pattern generators. The central pattern generators transmit the basic pattern of your breath to the phrenic nerve that in turn causes your diaphragm to rhythmically contract and relax. (Boron & Boulpaep, 2012)
The central pattern generators explain your basal rhythm of breathing, but how do we explain the wide range of rate, rhythm, and depth that characterizes your everyday breathing? To explain this variabilty, we must delve deeper into the brainstem/“CPU.”
There are many different influences on the breath, but we will limit our discussion to two. Regulatory processes are roughly split into central and peripheral sources. “Central” refers to regulatory signals that originate from the central nervous system (spinal cord, brainstem/“CPU,” limbic system, and neocortex). “Peripheral” identifies those regulatory signals that originate somewhere outside of the central nervous system.
The two sources of regulation that we will discuss are chemoreceptors and slowly adapting pulmonary stretch receptors (SARs). We will first look at the chemoreceptors.
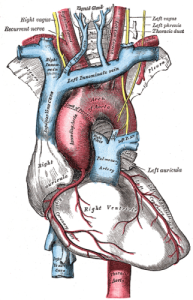
AORTIC ARCH “Gray505“. Licensed under Public Domain via Wikimedia Commons.
The chemoreceptors that we are concerned with today detect O2 and CO2 concentration in the blood. Central chemoreceptors detect CO2 while peripheral chemoreceptors detect O2.
There are two primary anatomical locations for peripheral chemoreceptors: the aortic bodies, as they are known, are located in the arch of the aorta while the carotid bodies are located at the bifurcation of the internal and external carotid arteries. The aorta is the largest artery in your body, receiving blood pumped directly from the heart. The carotid arteries (there are two) are located on either side of the neck and produce the pulse that you can feel by placing your fingers under the corner of your jawbone. We will return to the aortic and the carotid bodies later when we discuss baroreceptors.
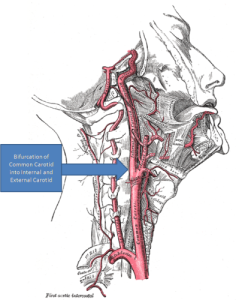
CAROTID ARTERY BIFURCATION “Gray’s Anatomy with markup showing carotid artery bifurcation” by Gray513.png: Henry Grayderivative work: Skoch3 (talk) – Gray513.png. Licensed under Public Domain via Wikimedia Commons.
These peripheral chemoreceptors primary detect low O2 in the blood, signaling the brainstem/“CPU” to increase your respiratory rate in order to take in more O2. The aortic body chemoreceptors send their low O2 signal along the vagus nerve, the 10th cranial nerve (there are 12 paired cranial nerves that originate in the brainstem/“CPU”). The carotid body chemoreceptors transmit their low O2 signal along the glossopharyngeal nerve, the 9th cranial nerve. The vagus and glossopharyngeal nerves are paired (there are two of them), but are normally referred to in the singular. (Boron & Boulpaep, 2012)
The low O2 signals are processed in a part of the brainstem/“CPU” known as the medulla. The medulla then signals the respiratory centers (also in the brainstem/“CPU”) to increase the rate of your breathing, attempting to make up for your O2 deficit.
The central chemoreceptors are also located in the part of the brainstem/“CPU” known as the medulla. Instead of responding to low O2 however, the central chemoreceptors respond to high CO2. Elevations in CO2 can be caused by any number of mechanisms, but no matter the source, excess CO2 can be “blown off” during exhalation if you increase your respiratory rate.
The central chemoreceptors in the medulla are in close proximity to the fluid known as cerebrospinal fluid (CSF) that encases your central nervous system. CO2 combines with water in the CSF to form carbonic acid, which in turn lowers the pH of the CSF, increasing acidity. This increase in acidity is what the central chemoreceptors detect. (Boron & Boulpaep, 2012)
Now we are able to appreciate that both low O2 and high CO2 can increase your respiratory rate. Derangements in blood O2 and CO2 can be caused by any number of mechanisms, but for the purposes of our discussion let’s imagine the most typical: holding your breath.
As you hold your breath, blood continues to pump throughout your body, depositing its vital O2 in various tissues while retrieving CO2 byproducts for exhalation. However, now that you are not breathing, the lungs are not taking in fresh O2 or exhaling CO2 so your circulating blood begins to accumulate CO2 and run low on O2.
Your peripheral chemoreceptors sense the low O2 while your central chemoreceptors detect the high CO2. Both signals trigger the brainstem/“CPU” to increase your rate of breathing. At a certain point you can’t consciously resist the urge to breathe, and you take a long, deep breath.
If you imagine the stress of holding your breath, then you might be able to predict the final detail of the chemoreceptor reflex arc. Along with an increase in respiratory rate, low O2 or high CO2 and the subsequent triggering of the chemoreceptor axis also increases the output of the sympathetic nervous system. (Boron & Boulpaep, 2012)
If we recall from previous discussions, the sympathetic nervous system (SNS), acts as the “gas” in your body, dialing up your heart rate and blood pressure, dilating your bronchioles (airways), and diverting blood to your skeletal muscles for your fight-or-flight response.
Now that we have discussed the role of chemoreceptors in the regulation of breathing let’s turn to the second source of regulation: slowly adapting pulmonary stretch receptors (SARs).
SARs are triggered during your inhalation as the lungs expand and stretch. The signals are transmitted along your vagus nerve (10th nerve) to your brainstem/“CPU.” This signal inhibits your drive to inhale further, communicating the need to begin exhaling, thus preventing overinflation of the lungs. As your inhalation and subsequent lung expansion increases, so too does the signal from the SARs. This reflex is known as the Hering-Breuer reflex. The SARs have a wide range of inhibitory effects in the brainstem/“CPU” that we will examine later in our discussion of the heart.
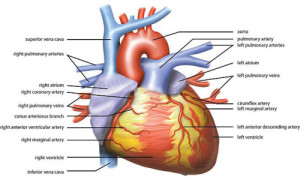
HEART ANATOMY “Wiki Heart Antomy Ties van Brussel” by Tvanbr – Own work. Licensed under CC BY-SA 3.0 via Wikimedia Commons.
Now that we have examined the lungs let’s turn to the heart.
The rate and rhythm of your heartbeat is set by a group of cells in the right atrium of your heart known collectively as the sinoatrial node (SA node). The SA node is where “sinus rhythm,” the normal rhythm of your heart, gets its name. Without outside influence the SA node would cause your heart to beat at around 100 beats per minute. Considering that the average heart rate is somewhere around 70-80 beats per minute there is obviously more to this story than the SA node (athletes tend to have a resting heart rate closer to 50 or 60).
The SA node receives signals from both the sympathetic nervous system, SNS/“Gas,” and its counterpart the parasympathetic nervous system, PSNS/“Brake.” If we remember from previous discussions, the SNS/“Gas” and PSNS/“Brake” represent a sort of internal yin and yang. Each system is never all the way off or all the way on; homeostasis depends on a balancing act between the two.

SNS/”GAS” & PSNS/”BRAKE” “Brake and Gas pedals of Enzo Ferrari” by Raiko – Own work. Licensed under CC BY-SA 3.0 via Wikimedia Commons.
In the case of the heart, the SNS/“Gas” speeds up the rate of the heart and causes your vasculature to constrict, raising blood pressure. The PSNS/“Brake” slows down the rate of the heart and causes mild vasculature dilation.
The PSNS/“Brake” signals are communicated to the heart by the vagus nerve. As we are beginning to see, the vagus nerve is a tremendously busy cranial nerve. Vagus actually means “wandering” in Latin to reflect its wide ranging inputs throughout the body.
The SNS/“Gas” signals are sent along thoracic spinal nerves to the SA node.
For our discussion we will primarily consider the rate effects of the PSNS/“Brake” courtesy of the vagus nerve. The vagus nerve requires less time to respond and recover than the SNS/“Gas.” In fact the delay from stimulation to heart rate modulation is about 5 times longer for the SNS/“Gas” than for the PSNS/“Brake” (Berntson, Cacioppo, & Quigley, 1993).
All of this is to say that for the purposes of examining our slow, deep breath we will ignore the SNS/“Gas” input on heart rate, instead focusing on the PSNS/“Brake” input from the vagus nerve.
In our now lengthy quest to understand our breathing we must turn now to the role of baroreceptors in regulating our heart rate and blood pressure.
The baroreceptors of interest for today’s discussion are groups of cells located in the same anatomical neighborhoods as the chemoreceptors that we considered in our earlier dialogue: just superior to the carotid bifurcations in the carotid sinus and in the arch of the aorta. Baroreceptors, derived from the Greek baros for “weight,” are sensitive to the “weight” of your blood (blood pressure).
Baroreceptors are activated when they detect an increase in blood pressure. The signal from the aortic arch travels along the vagus nerve while the signal from the carotid sinus is relayed to the brainstem/“CPU” by the glossopharyngeal (9th) nerve. These signals arrive at the part of the brainstem/“CPU” discussed earlier known as the medulla.
A pair of nuclei known as the nucleus tractus solitarii (NTS) within the medulla receives these high blood pressure signals from the vagus and glossopharyngeal nerves. The high blood pressure signal is then passed along to two more paired nuclei, also located in the medulla, known as the dorsal motor nucleus of the vagus and the nucleus ambiguus.
These nuclei then send a signal back out to the body along the vagus nerve to increase PSNS/“Brake” activity at the SA node, slowing the heart rate. At the same time, the NTS sends an inhibitory signal to the vasomotor area in the medulla that, when active, enhances the SNS/“Gas.” Thus, the NTS inhibits the SNS/“Gas” in response to high blood pressure.
In summary, high blood pressure is relayed to the brainstem/“CPU” which causes increased PSNS/“Brake” activity in the heart and decreased SNS/“Gas” in the vasculature with the net result being a decrease in heart rate and a decrease in blood pressure.
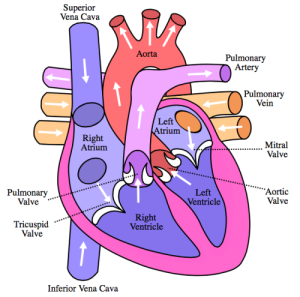
FLOW OF BLOOD THROUGH HEART “Diagram of the human heart (cropped)” by Own work. Licensed under CC BY-SA 3.0 via Wikimedia Commons.
Now let’s examine how the blood pressure changes during the respiratory cycle. As you inhale and your diaphragm contracts down into your abdominal cavity, the volume of your thoracic cavity increases. This increase in volume increases the negative intrathoracic pressure within the chest. This increased negative pressure acts as a vacuum, sucking blood into the highly compliant vasculature of the lungs, right side of the heart, and the venous system. The relative lack of blood to the left ventricle (output center of the heart) decreases stroke volume (amount of blood ejected during each heartbeat), which decreases blood pressure.
As you exhale and your diaphragm relaxes intrathoracic pressure normalizes, replenishing the relative deficit of blood in the left ventricle. This replenishment increases stroke volume and normalizes blood pressure.
Thus, as you inhale your blood pressure decreases slightly and as you exhale your blood pressure increases relative to the inhalation decrease.
What does this phasic change in blood pressure during normal respiration mean for the aforementioned neural reflexes?
The relative decrease in blood pressure during inhalation decreases your baroreceptor firing rate thereby releasing the PSNS/“Brake” hold on the SA node, leading to an increase in heart rate. Additionally, the decreased blood pressure drives an increase in the SNS/“Gas” outflow to the vasculature, in an attempt to increase blood pressure. The relative increase in blood pressure on exhalation has the opposite effect, increasing baroreceptor firing rate and signaling the brainstem/“CPU” to increase PSNS/“Brake” outflow to the SA node, decreasing heart rate. The increased blood pressure also produces a decrease in SNS/“Gas” outflow to the vasculature, in an attempt to decrease blood pressure. (Grossman & Taylor, 2007)
Due to changes in your blood pressure (among other mechanisms), when you inhale your heart rate increases and when you exhale your heart rate decreases.
This normal variation in your heart rate is called respiratory sinus arrhythmia (RSA); the mechanisms underlying RSA have been hotly debated for decades.
We must briefly return to your slowly adapting pulmonary stretch receptors (SARs) before continuing on to the final mechanism involved in the regulation of your breathing.
SARs, as we may remember, are triggered by the inhalational stretch of your lungs. The inhibitory signal from your SARs travel along the vagus nerve to tell your brainstem/“CPU” to stop inhaling and begin exhaling. As it turns out, the inhibitory signal from SARs has a wider range of effects than simply regulating your breathing.
The inhibitory signal from SARs during inhalation feeds into the nucleus tractus solitarii (NTS) within the brainstem/“CPU,” inhibiting the PSNS/“Brake” signals normally sent out along the vagus nerve back to the heart. Additionally, the SARs set up a “respiratory gate” that inhibits the maximum intensity of SNS/“Gas” signaling during inspiration (Eckberg, 2003).
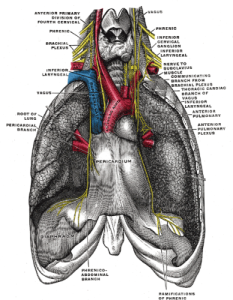
PHRENIC & VAGUS NERVES “Gray806“. Licensed under Public Domain via Wikimedia Commons.
The SA node in the heart is normally under tonic (constant) inhibition by the PSNS/“Brake” and the vagus nerve. And as we mentioned earlier, most of the heart rate variation in respiration is seen from either inhibition or excitation of this PSNS/“Brake” tonic inhibition. Thus, the decreased blood pressure baroreceptor reflex combined with the SARs conspires to inhibit the inhibitor (PSNS/“Brake”), increasing heart rate.
The final piece of our respiratory puzzle requires that we return to Dr. MacLean’s triune brain and examine the influence of the limbic system and the neocortex on the brainstem/“CPU.”
The brainstem/“CPU” does not operate in isolation, instead it receives input from many different “higher” (limbic and neocortex) and “lower” (vagus and glossopharyngeal nerves) sources. Until now we have primarily dealt with the “lower” influences, the more or less unconscious half of the equation. Now let’s turn to the subconscious and conscious factors.
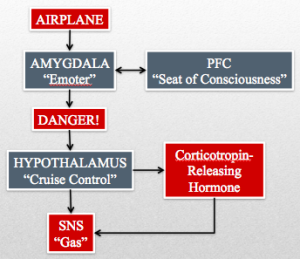
Maybe you’re afraid of flying. Despite this fear you muster up the courage to purchase a plane ticket to a far away tropical isle. On the day of your flight you are making your way down the cramped aisle of your plane, listening to the hum of the engines, and smelling the staleness of the recycled air. Panic begins to well up in your throat as you take your seat and fasten your seatbelt. Without me having to tell you anything more, you can already inform me that your heart is racing and that you’re having a hard time catching your breath.
So I’ve given you a panic attack — that’s great — now what does it have to do with your slow, deep breath?
The raw data from the airplane triggers a fear response in your amygdala/“Emoter” (limbic system) and your prefrontal cortex PFC/“Seat of Consciousness.” Your amygdala/“Emoter” sends “danger” signals to your locus ceruleus, which has norepinephrine neuronal projections throughout the brain. One such projection from the locus ceruleus is to the paraventricular nucleus of the hypothalamus/“Cruise Control.” The hypothalamus/“Cruise Control” triggers a release of corticotropin-releasing hormone and stimulates the SNS/“Gas” by inhibiting the inhibition of the nucleus tractus solitarii (NTS) on the vasomotor area of the medulla. Corticotropin-releasing hormone begins a cascade that ends with the adrenal glands releasing the SNS/“Gas” enhancing hormone cortisol (stress hormone).
The PFC/“Seat of Consciousness” has a reciprocal relationship with the amygdala/“Emoter” that allows it to either enhance or silence the activity of the amygdala/“Emoter.” In the example of your panic attack, the conscious thoughts of impending doom only serve to spur on the amygdala/“Emoter’s” already raucous fear signal.
I don’t want to leave you stranded mid panic attack, but we must return to your slow, deep breath to clarify the implications of this now rather large puzzle.
So why did we have to examine the physiological version of War and Peace to understand something as “simple” as a slow, deep breath?
Hopefully I have made it abundantly clear just how complex the simple act of breathing is. And it is within this complexity that you can begin to appreciate the tremendous power of attending to your breath.
Before we begin our examination of slow, deep breathing, let me explain the exercise so that you might try it for yourself.
Most of the research that I examined for this article defined slow, deep breathing as a rate of 6 breaths per minute. The following is an exercise that aims to replicate this breathing rate. You can vary the details based on your personal comfort level.
Inhale through your nose for approximately 4 seconds and then exhale through your nose for about 6 seconds. There are many different inhale to exhale ratios depending on the breathing technique employed, but for our purposes the ratio is less important than simply ensuring that your exhalation is longer than your inhalation. Also, if you can extend this single breath for longer than 10 seconds this is also fine. Repeat the above exercise 6 times to modulate your respiratory rate to 6 breaths per minute.

SLOW, DEEP BREATH “Phra Ajan Jerapunyo-Abbot of Watkungtaphao.” by ผู้สร้างสรรค์ผลงาน/ส่งข้อมูลเก็บในคลังข้อมูลเสรีวิกิมีเดียคอมมอนส์ – เทวประภาส มากคล้าย – Captured by uploader.. Licensed under CC BY 3.0 via Wikimedia Commons.
Breathing slowly and deeply, emphasizing the extended length of your exhalation, you may begin to appreciate a sense of calm. As you bask in this calming sensation let us return to our discussion.
Let’s examine the physiological implications of a slow, deep breath step by step in the order that we presented each mechanism.
First, slow, deep breathing increases O2 delivery to your tissues and optimizes ventilation perfusion matching in your lungs. Slow, deep breathing also decreases the amount of CO2 you expel (less exhalations per minute) and thus increases the relative concentration of CO2 in the blood. This increased CO2 is only a relative increase and does not reflect a pathological mechanism that would require your brainstem/“CPU” to increase respirations.
With multiple slow, deep breaths the increased exposure of your central chemoreceptors to higher levels of CO2 decreases their sensitivity to CO2’s effects, reducing the relative SNS/“Gas” activity when exposed to CO2. Additionally, the improved tissue oxygenation decreases the “stress” on tissues, further decreasing the SNS/“Gas” and shifting the balance towards the PSNS/“Brake.” (Pal & Velkumary, 2004)
Both the signal intensity and duration of the slowly adapting pulmonary stretch receptors (SARs) increase because of the increased stretch of the lungs (from a deeper breath) and the longer inhalation (relative to normal). This enhanced SARs signal travels along the vagus nerve to the brainstem/“CPU” and the nucleus tractus solitarii (NTS). The SARs inhibitory signal in the NTS not only inhibits the PSNS/“Brake” outflow, but also limits the maximum intensity of the SNS/“Gas.”
Inhibitory signals in the brainstem/“CPU” regulate excitability of neuronal cells throughout the central nervous system and have been shown to enhance synchronization of tissues in the brain. And in particular, synchronization between the hypothalamus/“Cruise Control” and the brainstem/“CPU” has been show to enhance overall PSNS/“Brake” outflow. (Jerath, Edry, Barnes, & Jerath, 2006)
Returning to the regulation of heart rate and blood pressure during normal respiration, you will recall the notion of respirator sinus arrhythmia (RSA).
RSA describes the increase in heart rate during inspiration (as blood pressure decreases) and the decrease heart rate during expiration (as blood pressure increases).
You may recall that the inhibition of the vagus nerve and the PSNS/“Brake” during inspiration causes an increase in heart rate while the excitation of the vagus nerve during expiration slowed the heart rate back down. You will note that the PSNS/“Brake” is most active during the expiratory phase of your breathing. (Brown & Gerbarg, 2005)
Thus, the prolongation of your expiratory phase in the slow, deep breathing exercise produces a relative increase in your PSNS/“Brake” drive.
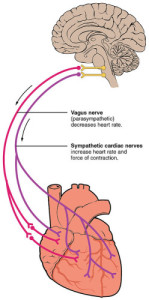
VAGUS NERVE & HEART “2032 Automatic Innervation” by OpenStax College – Anatomy & Physiology, Connexions Web site. http://cnx.org/content/col11496/1.6/, Jun 19, 2013.. Licensed under CC BY 3.0 via Wikimedia Commons.
Along with this relative increase in PSNS/“Brake” drive the normal physiological excitation and inhibition of the baroreceptor reflex arc is also prolonged with slower inhalation and exhalation respiratory phases. With more time in each phase of relative high and low pressure (inspiration and expiration), the baroreceptors are more capable of modulating the heart rate down and up to match the respective blood pressure. This enhancement of natural physiology increases the sensitivity of arterial baroreceptors. (Kaushik, Kaushik, Mahajan, & Rajesh, 2006)
The increased sensitivity of arterial baroreceptors increases the amplitude of your respiratory sinus arrhythmia (RSA). Because heart rate and thus RSA is determined primarily by the vagus nerve and the PSNS/“Brake,” the increased amplitude reflects a relative increase and flexibility of the PSNS/“Brake.” (Pal & Velkumary, 2004)
So to summarize: slow, deep breathing, through a wide array of mechanisms, increases PSNS/“Brake” activity and decreases SNS/“Gas” activity.
The final piece of the equation comes together when we return to Dr. MacLean’s most evolutionarily recent parts of the brain: the limbic system (amygdala/“Emoter” and hypothalamus/“Cruise Control”) and the neocortex (prefrontal cortex PFC/“Seat of Consciousness”).
During your panic attack on the airplane I mentioned that the brainstem/“CPU” has a reciprocal relationship between the limbic system and the PFC/“Seat of Consciousness.” Now I would like to expand on this.
The increase in PSNS/“Brake” drive in the brainstem/“CPU” is transmitted back up to the limbic system and the PFC/“Seat of Consciousness.” The inhibitory nature of the PSNS/“Brake” decreases the fearful activity of the amygdala and enhances the sense of wellbeing and stress reduction in the PFC/“Seat of Consciousness.” These mechanisms are thought to be what lie behind the subjective feeling of relaxation that occurs during a slow, deep breathing exercise. (Ankad, Herur, Patil, Shashikala, & Chinagudi, 2011)
Any number of meditative and yogic practices emphasize the return to slow, deep, diaphragmatic breathing. As I hope is clear from our lengthy discussion, the name or source of the given practice is less important than the common physiological mechanism that it taps in to.
Stephen Porges, MD is a psychiatrist at the University of North Carolina who has written a great deal about the aforementioned physiological mechanisms and the vagus nerve in particular. Dr. Porges developed his “Polyvagal Theory” to explain how the evolution of the vagus nerve and its role in the PSNS/“Brake” allowed early hominids to develop elaborate social behaviors.
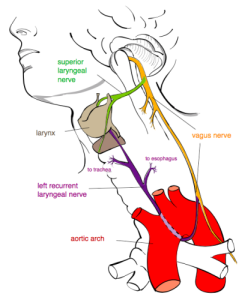
RECURRENT LARYNGEAL NERVE “Recurrent laryngeal nerve” by Jkwchui – Based on drawing by Truth-seeker2004. Licensed under CC BY-SA 3.0 via Wikimedia Commons.
Before we turn to the roles of the vagus nerve and the PSNS/“Brake” in human emotion, let’s briefly discuss spoken language. At some point during its evolutionary history, the vagus nerve gave rise to a branch known as the recurrent laryngeal nerve. The recurrent laryngeal nerve provides innervation for all but one of the muscles of the vocal cords. Dr. Porges suggests that the coevolution of mechanisms for improved control over vocalizations and the PSNS/“Brake” further reveals the central role of the vagus nerve in the develop of our social behavior. (Porges, 2011)
The entirety of the Polyvagal Theory is far beyond the scope of this article, but the theory has nonetheless, led to very interesting research on the role of the PSNS/“Brake” in human emotion.
Recent work has gone beyond the evolutionary speculation and has begun to establish a relationship between PSNS/“Brake” activity and emotional well-being. High vagal tone and the resultant elevation in resting PSNS/“Brake” tone are now known to be associated with trait happiness. Additionally, high resting PSNS/“Brake” tone predicts greater resilience in the face of stress; this association makes sense because stress induces SNS/“Gas” activity that is more effectively counterbalanced by a healthy influx of PSNS/“Brake” activity. (Kok & Fredrickson, 2010)
From these initial associations, researchers have demonstrated many associative links between higher resting PSNS/“Brake” activity and positive traits. For example, children with a higher resting PSNS/“Brake” tone are more resilient and perform better on cognitive tests. Furthermore, a parent’s resting PSNS/“Brake” tone predicts his or her child’s resting tone.
At this point you will likely be unsurprised to learn that the inverse relationship also exists between resting PSNS/“Brake” tone and emotion. Low resting PSNS/“Brake” tone (i.e. relatively unopposed SNS/“Gas”) is associated with negative emotionality (sadness, guilt, etc.). (Porges, Doussard-Roosevelt, & Maiti, 1994)
In a very real way, you are biologically programmed with the capacity to relax. Additionally, you are blessed with a will and consciousness capable of modulating your basic physiology. And as I hope I made clear over the preceding paragraphs, the practiced increase in PSNS/“Brake” (vagal) resting tone is associated with a whole litany of health benefits both for yourself and those around you.
Breathing is perhaps the most underappreciated physiological function of your body. I would wager that you are conscious of your breath for only a fraction of its total existence and yet it attends to you 20,000 times a day.
I hope that our discussion has encouraged you to look deeply into the wonder of your breathing and see the power of restorative change that it contains.
REFERENCES
Ankad, R. B., Herur, A., Patil, S., Shashikala, G. V., & Chinagudi, S. (2011). Effect of short-term pranayama and meditation on cardiovascular functions in healthy individuals. Heart views: the official journal of the Gulf Heart Association, 12(2), 58.
Berntson, G. G., Cacioppo, J. T., & Quigley, K. S. (1993). Respiratory sinus arrhythmia: autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology, 30(2), 183-196.
Boron, W. F., & Boulpaep, E. L. (2012). Medical Physiology, 2e Updated Edition: with STUDENT CONSULT Online Access. Elsevier Health Sciences.
Brown, R. P., & Gerbarg, P. L. (2005). Sudarshan Kriya yogic breathing in the treatment of stress, anxiety, and depression: part I-neurophysiologic model. Journal of Alternative & Complementary Medicine, 11(1), 189-201.
Butler, A. B., & Hodos, W. (2005). Comparative vertebrate neuroanatomy: evolution and adaptation. John Wiley & Sons.
Eckberg, D. L. (2003). The human respiratory gate. The Journal of Physiology, 548(Pt 2), 339.
Grossman, P., & Taylor, E. W. (2007). Toward understanding respiratory sinus arrhythmia: relations to cardiac vagal tone, evolution and biobehavioral functions. Biological psychology, 74(2), 263-285.
Jerath, R., Edry, J. W., Barnes, V. A., & Jerath, V. (2006). Physiology of long pranayamic breathing: neural respiratory elements may provide a mechanism that explains how slow deep breathing shifts the autonomic nervous system. Medical hypotheses, 67(3), 566-571.
Kaushik, R. M., Kaushik, R., Mahajan, S. K., & Rajesh, V. (2006). Effects of mental relaxation and slow breathing in essential hypertension. Complementary therapies in medicine, 14(2), 120-126.
Kok, B. E., & Fredrickson, B. L. (2010). Upward spirals of the heart: Autonomic flexibility, as indexed by vagal tone, reciprocally and prospectively predicts positive emotions and social connectedness. Biological psychology, 85(3), 432-436.
MacLean, P. D. (1990). The triune brain in evolution: Role in paleocerebral functions. Springer Science & Business Media.
Pal, G. K., & Velkumary, S. (2004). Effect of short-term practice of breathing exercises on autonomic functions in normal human volunteers. The Indian journal of medical research, 120(2), 115-121.
Porges, S. W. (2011). The Polyvagal Theory: Neurophysiological Foundations of Emotions, Attachment, Communication, and Self-regulation (Norton Series on Interpersonal Neurobiology). WW Norton & Company.
Porges, S. W., Doussard‐Roosevelt, J. A., & Maiti, A. K. (1994). Vagal tone and the physiological regulation of emotion. Monographs of the Society for Research in Child Development, 59(2‐3), 167-186.
Pramanik, T., Sharma, H. O., Mishra, S., Mishra, A., Prajapati, R., & Singh, S. (2009). Immediate effect of slow pace bhastrika pranayama on blood pressure and heart rate. The Journal of Alternative and Complementary Medicine, 15(3), 293-295.
Priban, I. P. (1963). An analysis of some short‐term patterns of breathing in man at rest. The Journal of physiology, 166(3), 425-434.
Seals, D. R., Suwarno, N. O., Joyner, M. J., Iber, C., Copeland, J. G., & Dempsey, J. A. (1993). Respiratory modulation of muscle sympathetic nerve activity in intact and lung denervated humans. Circulation research, 72(2), 440-454.
Shepherd, J. T. (1981). The lungs as receptor sites for cardiovascular regulation. Circulation, 63(1), 1-10.
Sinha, A. N., DeePAK, D., & Gusain, V. S. (2013). Assessment of the effects of pranayama/alternate nostril breathing on the parasympathetic nervous system in young adults. Journal of clinical and diagnostic research: JCDR, 7(5), 821.



![MRI by Nevit Dilmen / [CC-BY-SA-3.0 or GFDL]](/wp-content/uploads/2014/07/brain-color-e1406157479540-160x160.jpg)




What a great article! Thanks a lot!!
A lot of useful information. Alina
Thanks Alina, I’m so glad that you enjoyed it!
Cool article. I’ve always been interested in permanently improving my subconscious breathing patterns as I feel my natural breathing is very shallow. Interestingly enough I also suffer from anxiety.
Do you think it is possible to permanently change your subconscious breathing pattern or should I just focus on relaxation through breathing exercises?
Thanks for reading!
The human unconscious basal rate of respiration is entrained by the brainstem so a conscious effort to alter it would likely not be a great use of one’s time. Perhaps, the most benefit would be found just appreciating the primary experience of the practice.
Thank you again for reading!
Would constant, persistent practice in any way alter the subconscious pattern? I’ve been suffering from anxiety and bad body pains that I am 99% sure have to do with diaphragm and breathing, and would love to change that for the better! Not that I won’t try regardless of the answer.
Anyways, thank you so much for this great article.
Thank you for reading!
This question seems to be similar to a previous question. As I noted in my previous answer, the human basal respiratory rate tends to be entrained by the brainstem. That being said, physical exercise can theoretically lower basal heart rate as well as other physical parameters. Thanks again for reading!
Great read. I think that continued practice of slow breathing can “reprogram” a bad habit of shallow breathing.
Thanks for reading and taking the time to comment!
Thank you for this deep read. Just came across your web and found it really useful. As a meditation and yoga practitioner/researcher this article has helped me to better understand the mechanism of deep breathing.
Thank you for reading and for your support! I’m so glad that the article was helpful!
Dear Matt heartful thanks , your article is really wonderful and it has touched the sky in area of mindfulness and anxiety disorder. I find your breathing and mindfulness very comforting. But which is better ? To observe breath or take deep mindful breaths or both or some form of mantra . Similarly deep breathing is slightly difficult while standing but supine position it is good . Can I start with exhalation before I take a deep breath or should simply inflate my lungs? Pls clarify. Thank you
The practice of mindfulness is a very personal exercise. Whatever feels sustainable and relaxing is likely the right method for you. I’m sorry I don’t have a more definitive answer, but I would suggest that the exercise of trial and error will provide excellent self-education for establishing a practice. Thanks for reading!
Thank you Matt, indeed trial and error is good in time of paralysis of Logic. Interestingly you have covered both the posts i.e mindfulness of breathing and taking a deep breath. A small doubt regarding exhalation, we say smooth and longer exhalation turns on parasympathetic especially in recoiling mode with minimal utilisation of intercoastals and abdominal muscles. If so does this parasympathetic state help in return of alertness which is probably not available from ordinary eye resting.Does deep breathing also alter brainwave pattern and aid in creativity by generating greater alpha wave coherence? pls try to provide impact of deep diaphragmatic breathing on generation of alpha waves
Thank you again for your comments and questions. I would recommend reading the following article for more details regarding the physiology of deep breathing: Jerath, R., Edry, J. W., Barnes, V. A., & Jerath, V. (2006). Physiology of long pranayamic breathing: neural respiratory elements may provide a mechanism that explains how slow deep breathing shifts the autonomic nervous system. Medical hypotheses, 67(3), 566-571.